| |
Research Highlights:
Precise Structures
HIV-1
Cholesterol
Peptides in Membranes
Rafts
Water Permeability Through Membranes
|
|
Questions: How does the HIV-1 virus enter the T-cell? How are the elastic properties of the host cell membrane changed by the fusion peptide FP23? Where is FP located in a mimic of the T-cell membrane? How does cholesterol control the position of FP23? What is the location of the trimer form of FP23? Does the trimer disorder the membrane? Where is another HIV peptide, the cholesterol-sequestering CRAC motif, located in a membrane? How does the CRAC peptide affect the membrane thickness? How are the elastic properties affected by the CRAC motif peptide?

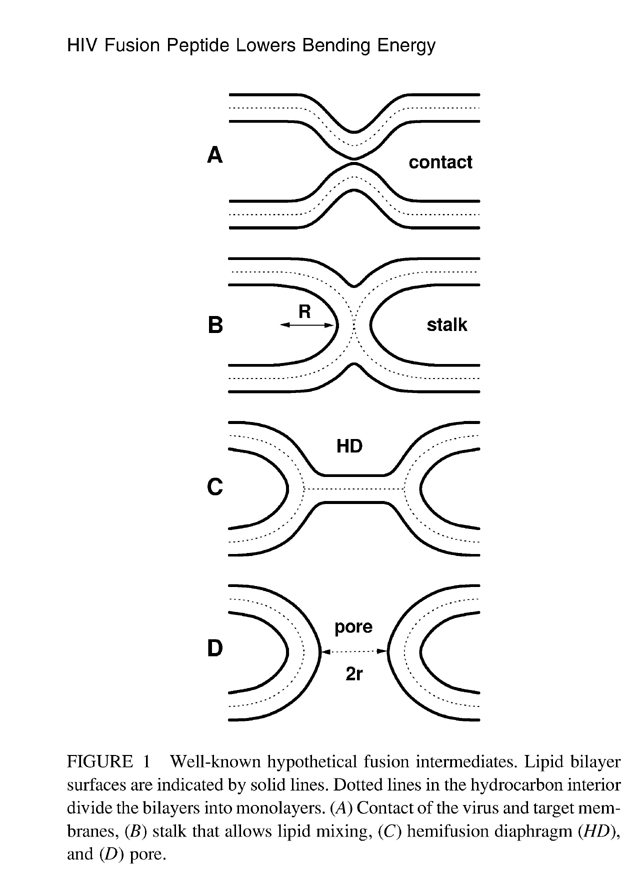
HIV-1 Fusion Peptide Decreases Bending Energy and Promotes Curved
Fusion Intermediates
Biophysical Journal Volume 93, September 2007 2048–2055
Stephanie Tristram-Nagle* and John F. Nagle*1
*Biological Physics Group, Department of Physics, and 1Department of Biological Sciences, Carnegie Mellon University, Pittsburgh, Pennsylvania 15213
a b s t r a c t A crucial step in human immunodeficiency virus (HIV) infection is fusion between the viral envelope and the T-cell membrane, which must involve intermediate membrane states with high curvature. Our main result from diffuse x-ray scattering is that the bending modulus Kc is greatly reduced upon addition of the HIV fusion peptide FP-23 to lipid bilayers. A smaller bending modulus reduces the free energy barriers required to achieve and pass through the highly curved intermediate states and thereby facilitates fusion and HIV infection. The reduction in Kc is by a factor of 13 for the thicker, stiffer 1,2-sn-dierucoylphosphatidylcholine bilayers and by a factor of 3 for 1,2-sn-dioleoylphosphatidylcholine bilayers. The reduction in Kc decays exponentially with concentration of FP-23, and the 1/e concentration is, 1 mol% peptide/lipid, which is well within the physiological range for a fusion site. A secondary result is, when FP-23 is added to the samples which consist of stacks of membranes, that the distance between membranes increases and eventually becomes infinite at full hydration (unbinding); we attribute this both to electrostatic repulsion of the positively charged arginine in the FP-23 and to an increase in the repulsive fluctuation interaction brought about by the smaller Kc. Although this latter interaction works against membrane fusion, our results show that the energy that it requires of the fusion protein machinery to bring the HIV envelope membrane and the target T-cell membrane into close contact is negligible. |
 |
 |
We welcome you to read the article - click here.

HIV Fusion Peptide Penetrates, Disorders, and Softens T-Cell Membrane Mimics
Journal of Molecular Biology Volume 402, 2010, 139–153
Stephanie Tristram-Nagle1, Rob Chan1, Edgar Kooijman2, Pradeep Uppamoochikkal1, Wei Qiang3, David P. Weliky3 and John F. Nagle1,4
1Biological Physics Group, Department of Physics, Carnegie Mellon University, Pittsburgh, PA,
2Department of Biological Sciences, Kent State University, Kent, OH,
3Department of Chemistry, Michigan State University, East Lansing, MI,
4Department of Biological Sciences, Carnegie Mellon University, Pittsburgh, PA |
abstract
This work investigates the interaction of N-terminal gp41 fusion peptide (FP) of human immunodeficiency virus type 1 (HIV-1) with model membranes in order to elucidate how FP leads to fusion of HIV and T-cell membranes. FP constructs were (i) wild-type FP23 (23 N-terminal amino acids of gp41), (ii) water-soluble monomeric FP that adds six lysines on the C-terminus of FP23 (FPwsm), and (iii) the C-terminus covalently linked trimeric version (FPtri) of FPwsm. Model membranes were (i) LM3 (a T-cell mimic), (ii) 1,2-dioleoyl-sn-glycero-3-phosphocholine, (iii) 1,2-
dioleoyl-sn-glycero-3-phosphocholine/30 mol% cholesterol, (iv) 1,2-dierucoyl-
sn-glycero-3-phosphocholine, and (v) 1,2-dierucoyl-sn-glycero-3-phosphocholine/
30 mol% cholesterol. Diffuse synchrotron low-angle x-ray
scattering from fully hydrated samples, supplemented by volumetric data, showed that FP23 and FPtri penetrate into the hydrocarbon region and cause membranes to thin. Depth of penetration appears to depend upon a complex combination of factors including bilayer thickness, presence of cholesterol, and electrostatics. X-ray data showed an increase in curvature in hexagonal phase 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine, which further indicates that FP23 penetrates into the hydrocarbon region rather than residing in the interfacial headgroup region. Low-angle x-ray scattering data also yielded the bending modulus KC, a measure of membrane stiffness, and wide-angle x-ray scattering yielded the Sxray orientational order parameter. Both FP23 and FPtri decreased KC and Sxray considerably, while the weak effect of FPwsm suggests that it did not partition strongly into LM3 model membranes. Our results are consistent with the HIV FP
disordering and softening the T-cell membrane, thereby lowering the activation energy for viral membrane fusion. |
 |
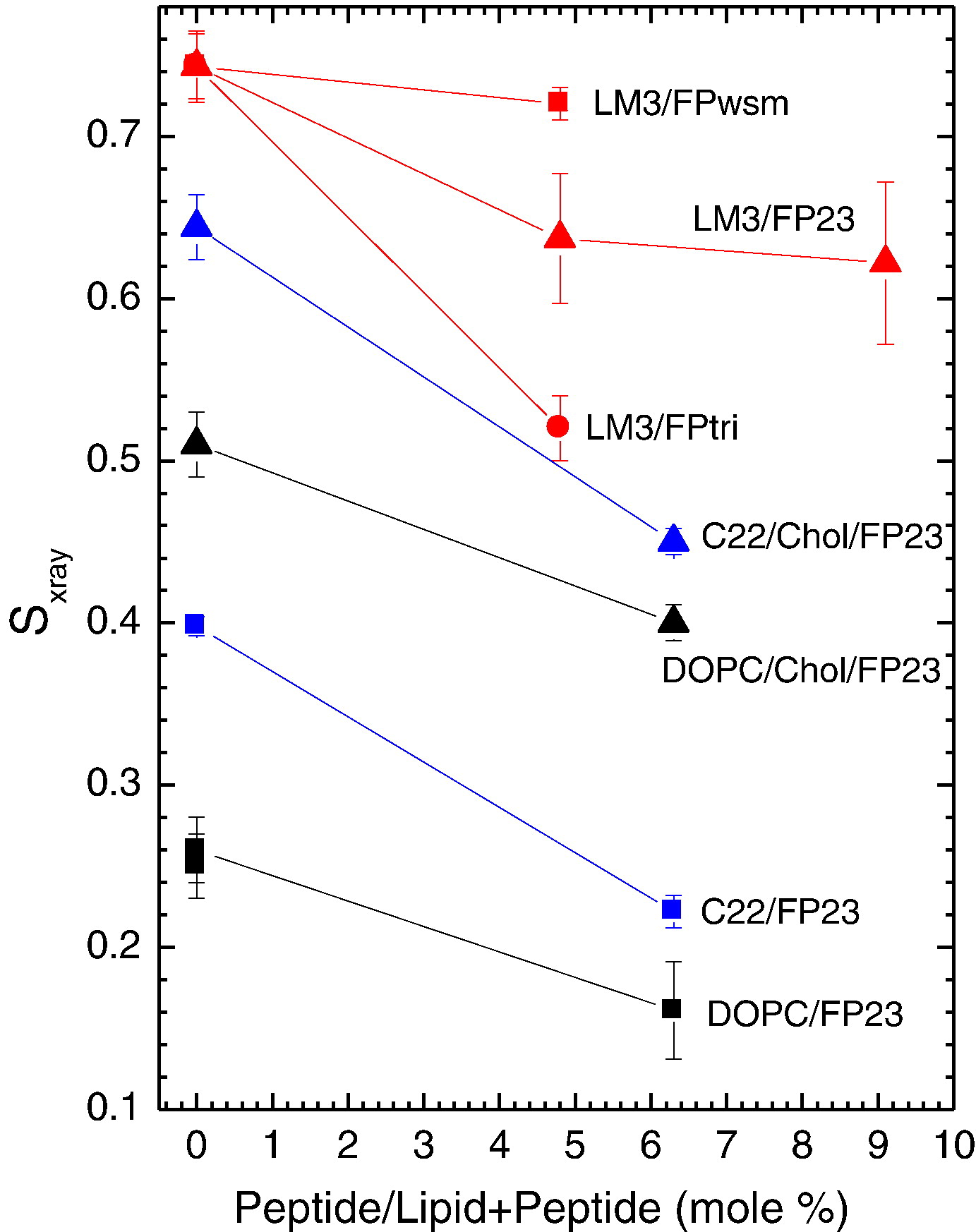 |
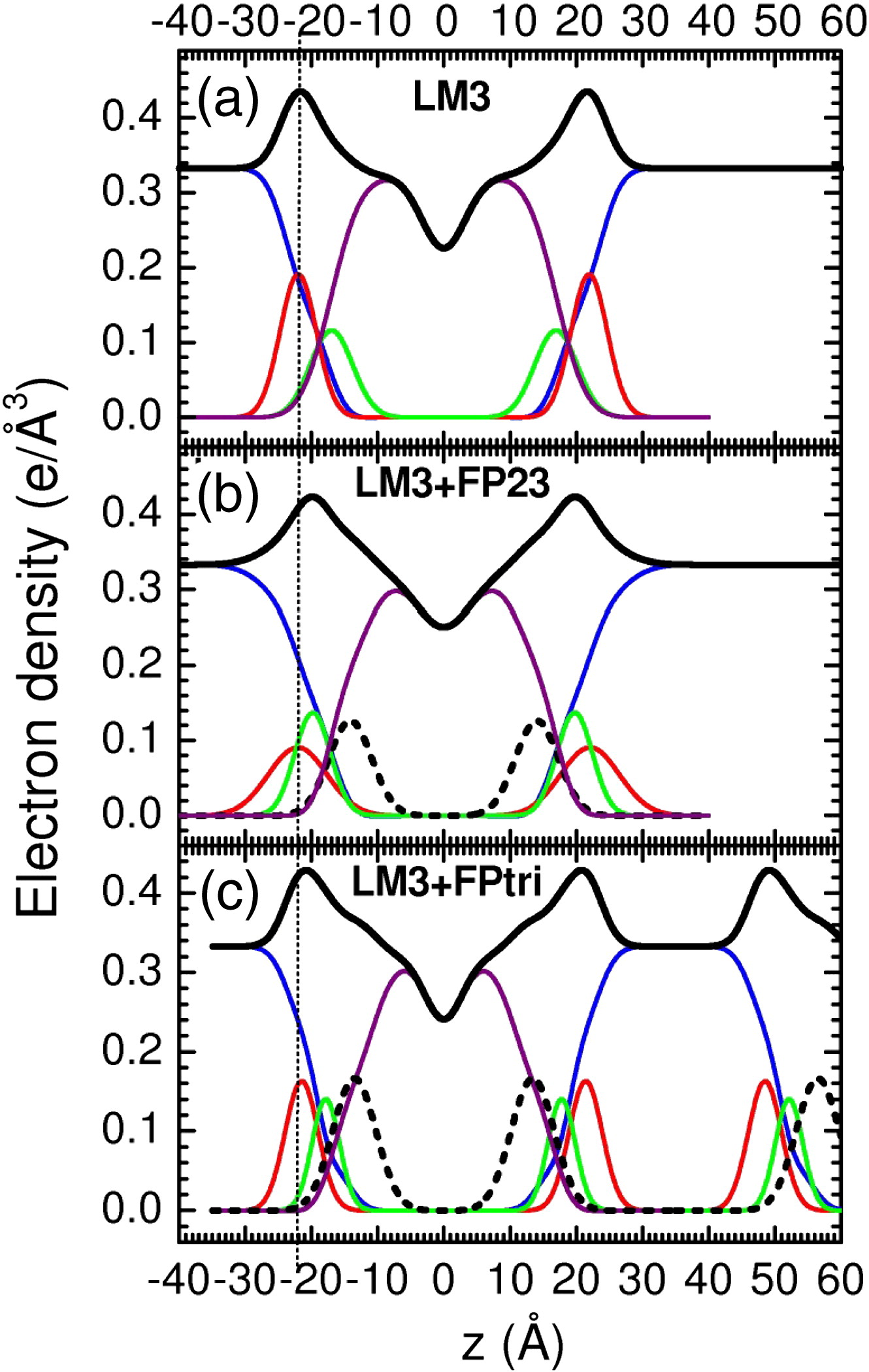
Figure 1. Electron density versus distance from the bilayer center z from samples of (a) LM3, (b) 4.8 mol% FP23/LM3, and (c) 1.6 mol% FPtri/LM3. Line colors: Black is total
electron density, blue is water, red is phosphate plus choline or other average headgroup moiety, green is glycerol– carbonyl, purple is hydrocarbon region including cholesterol when present, and black dotted line is peptide. (c) shows part of a second adjacent bilayer at large values of z, and the presence of a large water spacing
between adjacent bilayers is emphasized in all panels. |
Figure 2. Sxray order parameters as a function of increasing peptide concentration; 1.6mol% FPtri/LM3 is plotted at the equivalent native amino acid concentration, 4.8mol% FPtri/
LM3, for appropriate comparison. Lines are to guide the eye.
|
We welcome you to read the article - click here.
|
 CRAC motif peptide of the HIV-1 gp41 protein thins SOPC CRAC motif peptide of the HIV-1 gp41 protein thins SOPC
membranes and interacts with cholesterol
Biochimica et Biophysica Acta 1778 (2008) 1120–1130
Alexander I. Greenwood1, Jianjun Pan1, Thalia T. Mills1, John F. Nagle 1,2,
Richard M. Epand3, Stephanie Tristram-Nagle1
1Biological Physics Group, Physics Department, Carnegie Mellon University, 5000 Forbes Avenue, Pittsburgh, PA 15213, USA 2Department of Biological Sciences, Carnegie Mellon University, 4000 Fifth Avenue, Pittsburgh, PA 15213, USA 3Department of Biochemistry/ Biomedical Sciences, McMaster University, 1200 Main Street West, Hamilton, ON, Canada
a b s t r a c t
This study uses low-angle (LAXS) and wide-angle (WAXS) X-ray synchrotron scattering, volume measurements and thin layer chromatography to determine the structure and interactions of SOPC, SOPC/cholesterol mixtures, SOPC/peptide and SOPC/cholesterol/ peptide mixtures. N-acetyl-LWYIK-amide (LWYIK) represents the naturally-occurring CRAC motif segment in the pretransmembrane region of the gp41 protein of HIV-1, and N-acetyl-IWYIK-amide (IWYIK), an unnatural isomer, is used as a control. Both peptides thin the SOPC bilayer by ~3Å, and cause the area/unit cell (peptide+SOPC) to increase by ~9Å^2 from the area/lipid of SOPC at 30 °C (67.0±0.9 Å^2). Model fitting suggests that LWYIK's average position is slightly closer to the bilayer center than IWYIK's, and both peptides are just inside of the phosphate headgroup. Both peptides increase the wide-angle spacing d of SOPC without cholesterol, whereas with 50% cholesterol LWYIK increases d but IWYIK decreases d. TLC shows that LWYIK is more hydro-phobic than IWYIK; this difference persists in peptide/SOPC 1:9 mole ratio mixtures. Both peptides counteract the chain ordering effect of cholesterol to roughly the same degree, and both decrease Kc, the bending modulus, thus increasing the SOPC membrane fluidity. Both peptides nucleate crystals of cholesterol, but the LWYIK-induced crystals are weaker and dissolve more easily.
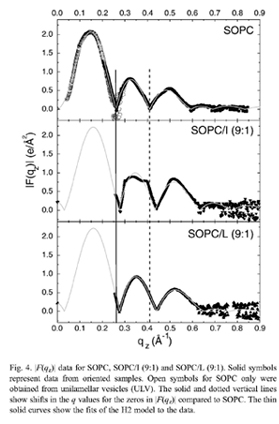 |
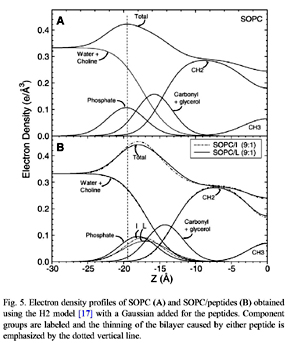 |
We welcome you to read the article - click here. |
| |
|
|
|
|
HIV-1
